WPBG-300
Cas : 1801263-71-7

Plastics manufacturing
Photocurable
- Shows high stability (pot life) in monomer.
- Generates a strong base, biguanide (pKbH=31.8), upon irradiation.
- Can be exposed with sensitizer at 365 nm and longer wavelength.
- Can be employed with cross-linking agents such as thiol or anhydride.
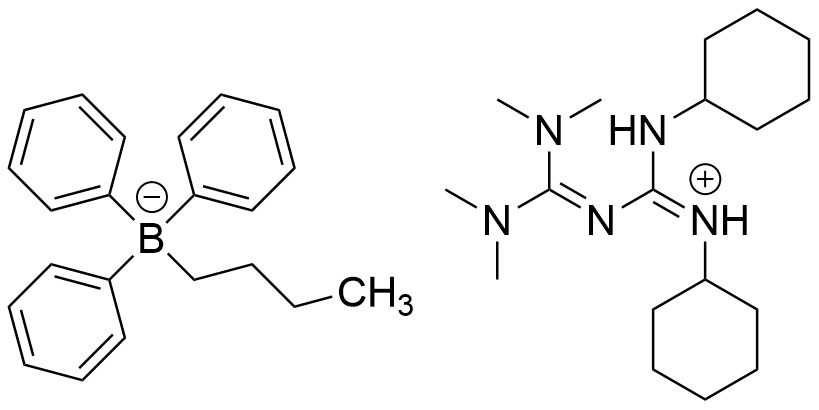
| Chemical name | 1,2-Dicyclohexyl-4,4,5,5-tetramethylbiguanidium n-butyltriphenylborate |
|---|---|
| CAS RN® | 1801263-71-7 |
| Molecular weight | 621.75 |
Physical characteristics
| Color Appearance |
white powder |
|---|---|
| Melting point/freezing point | 115℃ |
| Solubilities | water : slightly soluble. |
| TG-DTA | weight loss from 203℃ |
| Solubility | (g/solv. 100 g)
|
UV(0.02 mg/mL in CH3CN)
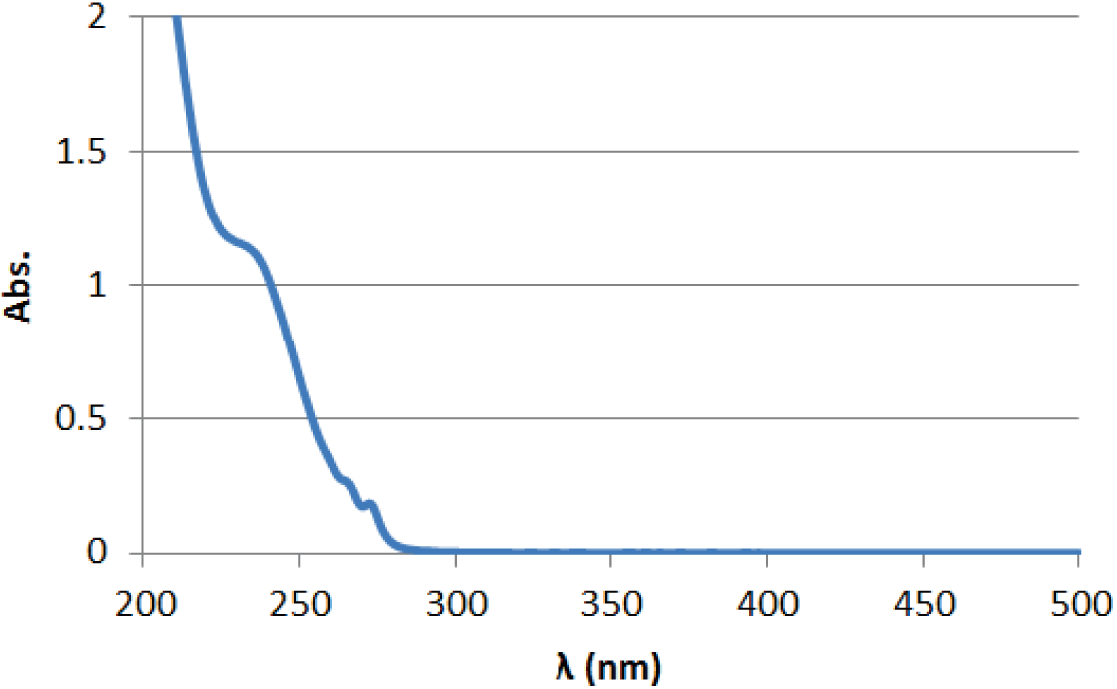
Absorption maximum
197 nm(ε=79000) 254 nm(ε=14000) 365 nm(ε=0)
Related laws and regulations
| TSCA | Not Listed |
|---|---|
| EINECS | Not Listed |
| REACH | Not Listed |
Packaging
- 5g
Mechanism of base generation
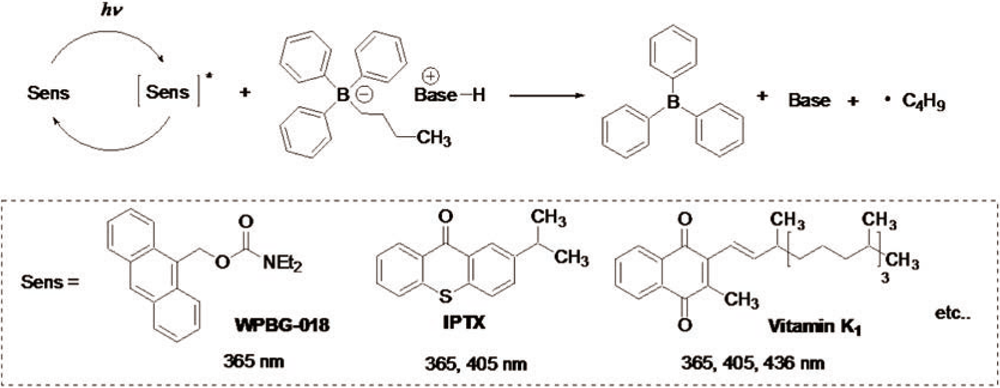
Example of Use 1Anionic UV curing of epoxy oligomer × polyfunctional thiol
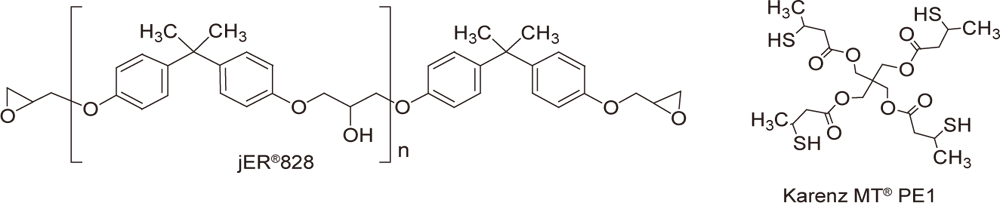
Conditions
| Preparation | Mix 1-5 parts of WPBG 300, 0.2-1 part of 2-isopropylthioxanthone (0.2 equivalent amount to WPBG), and 100 parts of jER®828 (epoxy equivalent of 185, Product of Mitsubishi Chemical Corporation), and heat or use a diluent to promote dissolution. Mix 70 parts of KarenzMT®PE1 (SH equivalent of 138.5, Product of Showa Denko K.K.) at room temperature. |
|---|---|
| Stability of composition as pot life (period in which the viscosity does not exceed the twice of the initial viscosity) | 1 month (10°C), 1 week (25°C), and 3 days (40°C) |
| Exposure | Irradiation for 10 seconds (Illuminance: 5mW/cm2 (254 nm), 100 mW/cm2 (365 nm), and 261 mW/cm2 (405 nm)) |
Example of Use 2Anionic UV curing of epoxy oligomer × acid anhydride
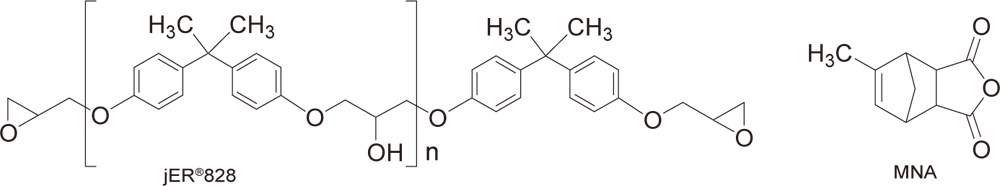
Conditions
| Preparation | Mix 3-10 parts of WPBG-300, 0.6-2 parts of 2-isopropylthioxanthone (0.2 equivalent amount to WPBG), and 100 parts of jER®828 (epoxy equivalent of 185, Product of Mitsubishi Chemical Corporation), and heat or use a diluent to dissolve. Mix 50 parts of methyl-5-norbornene-2,3-dicarboxylic anhydride (Product of FUJIFILM Wako Pure Chemical Corporation) at room temperature. |
|---|---|
| Stability of composition as pot life (period in which the viscosity does not exceed the twice of the initial viscosity) | 1 month (10°C), 1 week (25°C), and 3 days (40°C) |
| Exposure | Irradiation for 10 seconds (Illuminance: 5mW/cm2 (254 nm), 100 mW/cm2 (365 nm), and 261 mW/cm2 (405 nm)) |
Example of Use 3Anionic UV curing of episulfide
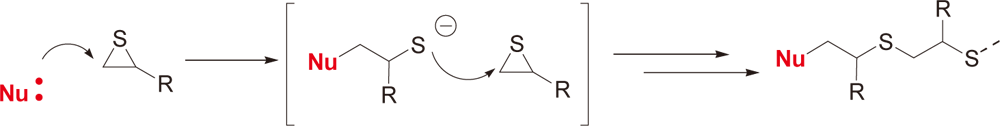
Conditions
| Preparation | Mix 1-5 parts of WPBG-300 and 0.2-1 part of 2-isopropylthioxanthone (0.2 equivalent amount to WPBG) in 20 parts of γ-butyrolactone and mix hydrogenated bisphenol A type episulphide (episulphide equivalent of 220). |
|---|---|
| Stability of composition as pot life (period in which the viscosity does not exceed the twice of the initial viscosity) | 1 month (10°C), 1 week (25°C), and 3 days (40°C) |
| Exposure | Irradiation for 10 seconds (Illuminance: 5mW/cm2 (254 nm), 100 mW/cm2 (365 nm), and 261 mW/cm2 (405 nm)) |
Contact us for more details.
We are waiting for questions and requests on products.

