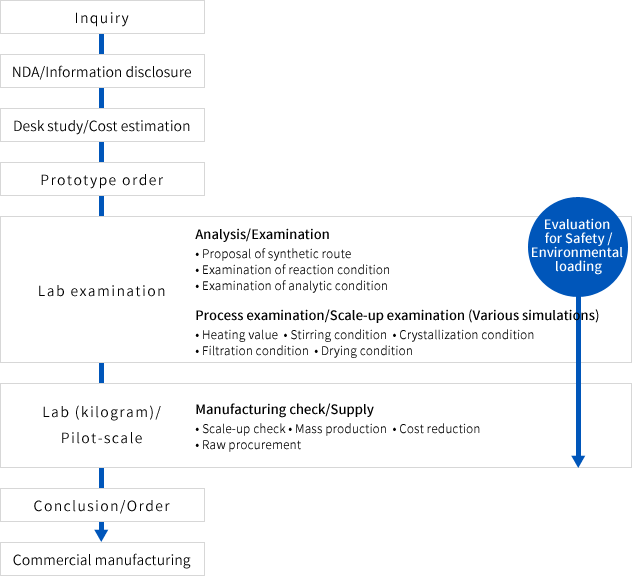
Flow from inquiry to commercial manufacturing for pharmaceutical products
The flowchart of custom manufacturing/synthesis is as follows. This flowchart is the only example.
We flexibly respond to the customer requirement. Please feel free to contact us.
Integrated value chain from procurement of raw materials to drug application.

Manufacturing achievements for pharmaceutical products
Approved Pharmaceutical Products
APIs:4 (Japanese market)
Starting substances:4 (Japanese market)
Intermediate:1 (FDA approved)
Pharmaceutical Products under the development
Clinical trial of APIs and intermediates: Numerous
Notes
- Proper containment measures are taken prior to manufacturing for substances with infectivity, strong pharmacological effect, or toxicity such as cytotoxic anti-cancer agents.
-
The following substances are not handled at current facilities.
1. Substances with strong sensitization such as penicillin and cephalosporin
2. Steroids
3. Non-pharmaceutical products with strong toxicity such as herbicides and pesticides
Please contact us about cost/case/technology/quality control
Inquiry formIntroducing examples of custom synthesis
Customer case studies
