It is recently reported that the radical reaction is used for the organic synthesis as a useful method of bond formation.
We have developed various azoic radical initiators, and we apply the process development for the organic synthesis with those unique initiators. We perform the effective and stereoselective reactions by selecting the radical initiators suitable for the reaction condition and/or reaction system of the compound requested from customers.
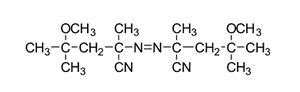
V-70
Example 1
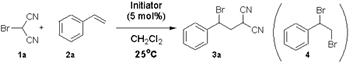
| Initiator | Time(hr) | Yield(%)of 3a | |
|---|---|---|---|
| AIBN | 24 | No Reaction | |
| BPO | 24 | No Reaction | |
| Et3B | 18 | 44 | |
| hv | 6 | 85 | |
| V-70 | 12 | 89 |
By using V-70, which is a polymerization initiator at low temperature, as a radical initiator, the reaction with bromo malononitrile which tends to decompose or disproportionate with heat proceeds effectively under room temperature.
Example 2
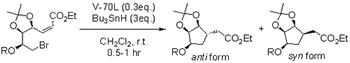
| Initiator | Solvent | Temp. (℃) | Yield (%) | Ratio (anti:syn) |
|---|---|---|---|---|
| hv | CH2Cl2 | refl. | 86 | 94:6 |
| Et3B | CH2Cl2 | -78 | 69 | 99:1 |
| AIBN | C6H6 | refl. | 80 | 86:14 |
| V-70L | CH2Cl2 | r.t. | 85 | 98:2 |
The above table shows the effect by each radical initiator with Z-bromopentanoic acid ester in the intramolecular cyclization reaction. Compared with the results with Et3B and AIBN, the result with V-70L shows higher yield and higher stereoselectivity of product materials.
Reference
Y. Kita, A. Sano, T. Yamaguchi, M. Oka, K. Gotanda, M. Matsugi,Tetrahedron Lett., 38, 3549 (1997)
M. Matsugi, K. Gotanda, C. Ohira, M. Suemura, A. Sano, Y. Kita,J. Org. Chem., 64, 6928 (1999)