What is high performance polymerization inhibitor?
- Mechanism of Polymerization Inhibition (Presumed)
- Comparison of Polymerization Inhibiting Effects
- Examples of Applications
- Thermal Polymerization Inhibiting Effect
- Polymerization Inhibiting Effect in Distillation (Heating and Reduced Pressure)
- Radical Polymerization Inhibiting Effect
- Measurement of the Induction Period of Radical Polymerization
- Propagation Inhibiting Effect in the Case of Abnormal Polymerization
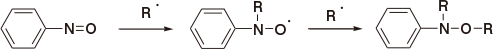
Mechanism of Polymerization Inhibition (Presumed)
In the cases of Q-1300 and Q-1301
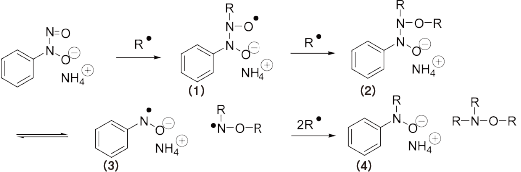
The nitroso group catches a radical in the system, and (1) is formed.
Attack of (1) to monomer causes a reaction with propagating radical to form a stable coupling product (2).
N-N cleavage of coupling product (2) proceeds at the temperature of 50°C or higher and forms a product (3).
Furthermore, the product (3) reacts with 2 radicals to form a product (4).
In the case of benzoquinone1)

In the case of nitroso compounds2)
Comparison of Polymerization Inhibiting Effects
- Purified styrene
- Concentration of polymerization inhibitor: 1,000 ppm
- Heating temperature: 120°C
- Under nitrogen gas flow
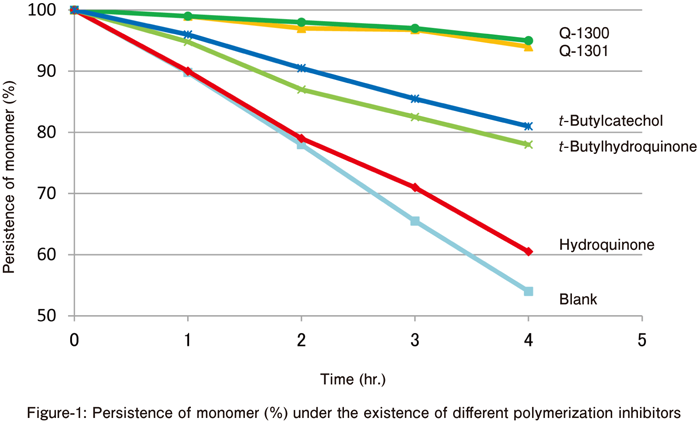
⇒ Q-1300 and Q-1301 show stronger polymerization inhibiting effect than other polymerization inhibitors at high temperature.
Examples of Applications
- Preservative stabilizer (monomers, UV inks, UV paints, and photosensitive resins).
- Prevention of polymerization during distillation of monomers.
- Prevention of polymerization during production of monomers and oligomers, and during heating/mixing of monomers.
- Suspension of polymerization when an abnormal polymerization is observed.
- Suspension of polymerization once the target polymerization rate is attained.
Thermal Polymerization Inhibiting Effect
Q-1300 and Q-1301 show an excellent inhibiting effect in thermal polymerization.
Acrylic acid3)
The formation level of polymerized product was comparatively-measured based on the change of relative viscosity of the solution which was heated for a certain period of time under the existence of polymerization inhibitor.
- 50% Acrylic acid water solution, polymerization inhibitor concentration of 1,000 ppm/monomer
- Heating temperature: 100°C
- Heating time: 8 hours
- Under nitrogen gas flow
Relative viscosity of acrylic acid water solution before and after heating
| Polymerization inhibitor | Relative viscosity (30°C) |
|---|---|
| No addition, before heating | 1.77 |
| Hydroquinone monomethyl ether | Unmeasurable because of high viscosity |
| Q-1300 | 1.77 |
Polyfunctional vinyl monomer4)
The thermal stability of polyfunctional vinyl monomer composition was measured.
Composition of monomer
![2-Propenoic acid, 2-methyl-, 1,1′-[(1-methylethylidene)bis[(2,6-dibromo-4,1-phenylene)oxy-2,1-ethanediyl]] ester CAS RN:67006 -39-7](image/about_img_05.png) |
60 part |
| Divinylbenzene | 20 part |
| Chlorostyrene(o-/p-=65/35) | 20 part |
- Heating temperature: 70°C
Comparison of thermal stability
| Polymerization inhibitor | Addition amount (ppm) | Result |
|---|---|---|
| Blank | - | Increase of viscosity after 5 hours |
| Hydroquinone monomethyl ether | 500 | Increase of viscosity and gelation after 3 hours |
| Q-1301 | 5 | No increase of viscosity and gelation, even after 15 hours |
Polymerization Inhibiting Effect in Distillation (Heating and Reduced Pressure)
Q-1300 and Q-1301 show an excellent inhibiting effect in distillation (heating and reduced pressure).
Acrylic acid5)
The inhibiting effect of the product when used for purification of acrylic acid by distillation was measured.
- Acrylic acid: 1 L
- Distillation temperature: 103°C/20 kPa
Comparison of polymerization inhibiting effect
| Polymerization inhibitor | Addition amount (ppm) | Result |
|---|---|---|
| Manganese acetate Hydroquinone monomethyl ether | 500 200 |
Formation of polymerized product was observed in the container and the distillation column after approximately 300 ml of distillate was obtained. |
| Manganese acetate Q-1300 | 20 20 |
Formation of polymerized product was not observed. |
Dimethylaminoethyl methacrylate6)
The effect of polymerization inhibitor was measured when synthesizing dimethylaminoethyl methacrylate by transesterification reaction, followed by separation and purification by distillation.
【Transesterification reaction】
- Methyl methacrylate: 750 g (7.5 mol)
- Dimethylaminoethanol: 268 g (3.0 mol)
- Pb: 12.4 g (0.06 mol)
- Reaction temperature: 65-70°C
- Reaction time: 4.5 hours
【Purification by distillation】
- Distillation temperature: 72°C/2.4 kPa - 63.5°C/0.7 kPa
Comparison of the inhibiting effects in transesterification reaction
| Polymerization inhibitor | Addition amount (g) | Yield (%) | Residue (g) | Remark |
|---|---|---|---|---|
| Hydroquinone monomethyl ether | 3.0 | - | - | Polymerizing during reactions |
| Q-1300 | 2.3 | 91.5 | 13.2 |
Radical Polymerization Inhibiting Effect
Vinyl chloride
During polymerization of vinyl chloride, stirring and cooling were stopped, and the change in temperature over time was measured after the addition of polymerization inhibitor.
- Vinyl chloride: Water = 1: 1.2, PVA 0.08%
- Radical polymerization initiator: V-65 (0.03%)
- Polymerization temperature: 56.5°C
- Q-1300: 100 ppm
- After five hours of polymerization, stirring and cooling in the system were stopped, and the polymerization inhibitor was added one minute later.
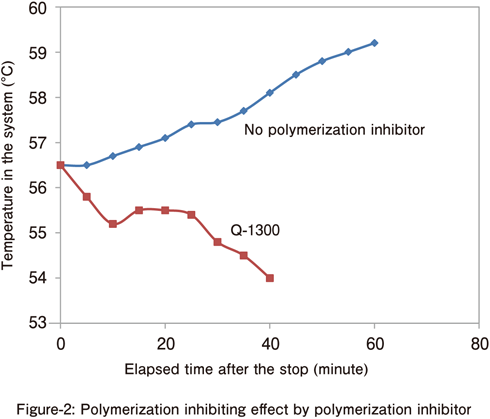
⇒ Q-1300 has an excellent inhibiting effect in suspension polymerization.
Measurement of the Induction Period of Radical Polymerization
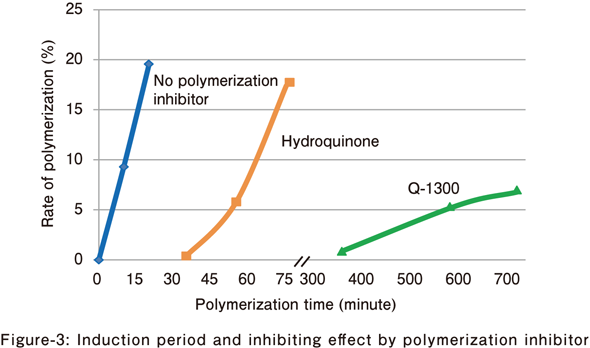
Sodium acrylate
The inhibiting effect of polymerization inhibitor in the water solution polymerization of sodium acrylate was evaluated.
- Monomer concentration: 20% (w/v)
- Polymerization inhibitor: 2,000 ppm/monomer
- Radical polymerization initiator for water solution:V-50 (0.4%/monomer)
- Polymerization temperature: 50°C
Acrylamide
The inhibiting effect of polymerization inhibitor in the water solution polymerization of acrylamide was evaluated.
- Monomer concentration: 50% (w/v)
- Polymerization inhibitor: 2,000 ppm/monomer
- Radical polymerization initiator for water solution:V-50 (1.0%/monomer)
- Polymerization temperature: 50°C
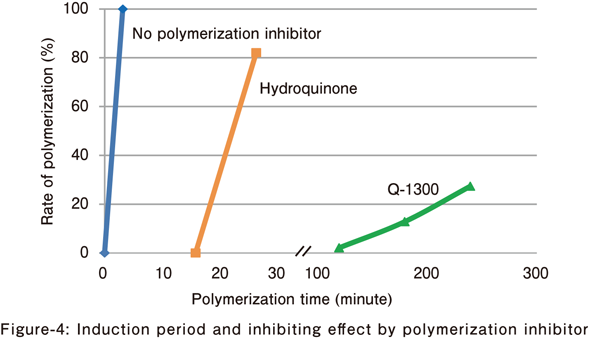
⇒ Q-1300 has a longer induction period and a stronger inhibiting effect as compared with other polymerization inhibitors.
Propagation Inhibiting Effect in the Case of Abnormal Polymerization
The product inhibits propagation of popcorn polymer* and shows a higher inhibiting effect than other inhibitors.
* Popcorn polymer: Generic name of less soluble and less meltable polymers formed by three-dimensional crosslinking of monomers during distillation.
Glycidyl methacrylate7)
We compared the growth of popcorn polymers under the condition of existence of polymerization inhibitor in the glycidyl methacrylate polymerization system.
- Glycidyl methacrylate: 30 part Methyl acrylate: 20 part Water:1,500 part
- Polymerization inhibitor: 1,000 ppm/monomer Polymerization temperature: 35°C Polymerization time:10 hours
- Polymerization initiator: APS-Na2SO3 Water-based redox polymerization
Inhibiting effect in the case of abnormal polymerization
| Inhibitor | Polymerization yield | Specific viscosity of polymer | Remark |
|---|---|---|---|
| Blank | 87.3 | 0.124 | Popcorn monomer 2.7 part formed |
| Q-1300 | 92.7 | 0.122 | No popcorn polymer formed |
Acrylic acid8)
Nucleus of popcorn polymer (SBR) was placed in the gas phase under the reflux condition of acrylic acid, and the change of mass of the popcorn polymer was measured.
- Reflux under reduced pressure (6.7 kPa), reaction time: 6 hours
Inhibiting effect in the case of abnormal polymerization
| Inhibitor | Addition amount (ppm) | Change of mass of popcorn nucleus (%) |
|---|---|---|
| Phenothiazine | 126 | +636 |
| Q-1300 | 102 | 0.0 |
Methyl methacrylate8)
Nucleus of popcorn polymer (SBR) was placed in the gas phase under the reflux condition of methyl methacrylate and the change of mass of the popcorn polymer was measured.
- Reflux under reduced pressure (6.7 kPa), reaction time: 6 hours
- The inhibitor was divided into six parts and added each part every an hour.
Inhibiting effect in the case of abnormal polymerization
| Inhibitor | Addition amount (ppm) | Change of mass of popcorn nucleus (%) |
|---|---|---|
| Phenothiazine | The same mole quantity as that of Q-1300 | +237 |
| Hydroquinone | The same mole quantity as that of Q-1300 | +243 |
| Q-1300 | 100 | +18 |
Contact us for more details.
We are waiting for questions and requests on products.

