Examples of reaction and application of cationic initiators
Polymerization Utilizing Acid
Cationic polymerization (chain polymerization)
[Ring-opening polymerization of epoxide and oxetane]
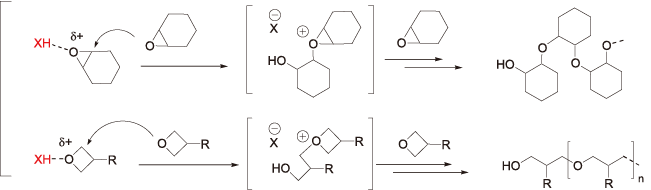
[Addition polymerization of vinyl ether]
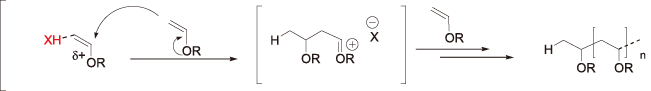
Since vinyl ether shows high reactivity, it is possible to initiate polymerization even with CF3SO3H. (In the case of iodonium, there is a large exotherm and a yellowing effect).
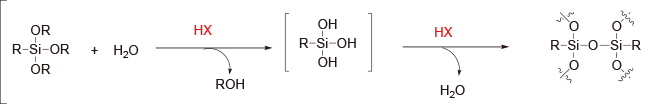
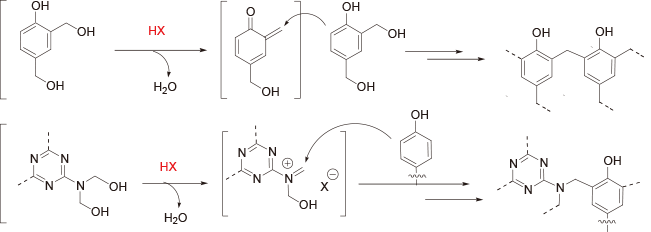
Condensation polymerization (Step-growth polymerization)
[Sol-gel method of alkoxylsilane]
[Condensation polymerization of hydroxymethyl group]
Strength and Applications of Typical Acids
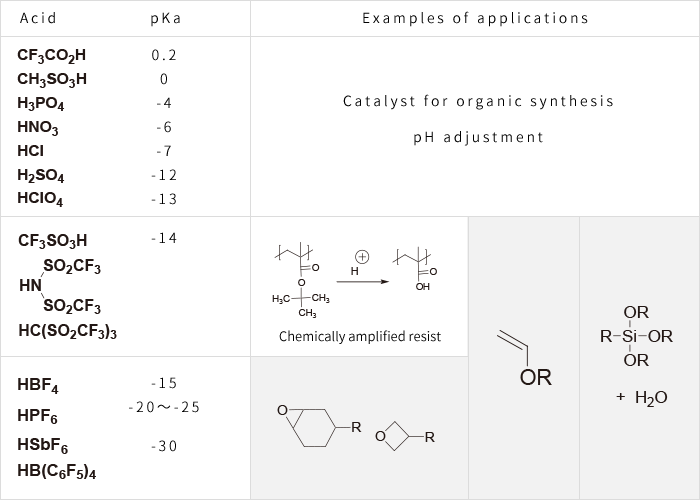
※ The colored part shows that the curing reaction is advanced by strong acid.
Difference of epoxy curing performance by the difference of anions
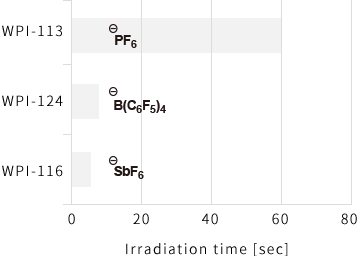
[Composition ratio]
Alicyclic epoxy CEL2021P / WPI-No. = 100 / 2
[Illuminance]
254nm : 5 mW/cm2
365nm : 100 mW/cm2
405nm : 217 mW/cm2
After leaving samples at the room temperature for 60 seconds, the irradiation time to attain pencil hardness H or more was measured, respectively.
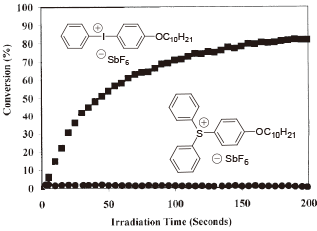
Various Sensitizers are Available for Iodonium
Among the onium salts, iodonium has a higher electron acceptance, and is easier to sensitize (customize) using additives.
Iodonium > Sulfonium > Ammonium
If a sensitizer with long wavelength absorption property is used, iodonium is able to be sensitized with irradiation of UV light ‒ visible light range (near infrared light) and to generate acid. Polycyclic aromatic compounds, heterocyclic compounds, dyes and metallic complexes which do not generate radicals with irradiation of light, are common sensitizers.
Comparison of photosensitivity with iodonium and sulfonium
When epoxy is cationic-polymerized under a light source with a 300 nm (or shorter) wavelength cut-off, and camphorquinone is used as a sensitizer, polymerization advances only in the case with iodonium.
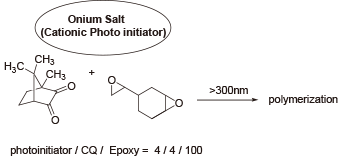
J. V. Crivello. M. Sangermano, J. Polym. Sci., Part A, Polym. Chem ., 2001 , 39 , 343-356
Compounds Accelerating Polymerization by Promoting the Decomposition of Iodonium
① 3,4-Dimethoxybenzyl alcohol
② Vinyl ether
③ N-Vinylcarbazole
Antioxidant which Improves the Preservation Stability of Composition.
Since the monomer of cationic polymerization is an ether , it is oxidized in air and forms peroxide. Peroxide is unstable and decomposes radically, while iodonium receives radicals easily and has a high reactivity. Addition of a radical polymerization inhibitor, such as BHT (dibutylhydroxytoluene), is known to improve stability.
| 2 functional oxetane | WPI-166 | BHT | Storage at 60℃ |
|---|---|---|---|
| 100 | 5 | Gelates in one day. | |
| 100 | 2 | 01 | Does not gelate in four days. |
Nobuaki Koike, “Photo Application Technology/Material Dictionary” P.167 (2006)
Contact us for more details.
We are waiting for questions and requests on products.

